- Author Jason Gerald gerald@how-what-advice.com.
- Public 2023-12-16 10:50.
- Last modified 2025-06-01 06:05.
Dilution is the process of making a concentrated solution more dilute. There are a variety of reasons why someone might want to do a dilution, ranging from serious to simple reasons. For example, biochemists dilute solutions from their concentrated form to make new solutions for use in experiments, whereas, on the other hand, bartenders often dilute liquor with soft drinks or juices to make cocktails tastier. The general formula for calculating dilution is C1V1 = C2V2, with C1 and C2 represents the initial and final concentrations of the solution, respectively, and V1 and V2 represents the volume.
Step
Method 1 of 2: Accurately Diluting Concentrates via the Dilution Equation

Step 1. Determine what you know and don't know
Dilution in chemistry usually means taking a small amount of a solution of which you know the concentration, then adding a neutral liquid (such as water) to make a new solution with a larger volume but lower concentration. This is very often done in chemistry laboratories, because, for the sake of effectiveness, reagents are often stored in very high concentrations, which are then diluted for use in experiments. Usually, in most real-world situations, you will know the concentration of your initial solution and the concentration or volume you want your final concentration to be, but not the volume of initial solution you need to obtain the final solution.
- However, in other situations (especially in school problems), you may need to find other pieces of the puzzle - for example, you may be given an initial volume and concentration, then asked to find the final concentration if you dilute a solution. according to the desired volume. In any case of dilution, it is helpful to take note of the known and unknown variables before starting.
-
Let's finish the example questions. Suppose we are asked to dilute a 5 M solution with water to make 1 L of a 1. solution mM. In this case, we know the concentration of our initial solution and the volume and final concentration we want, but not the amount of initial solution we need to add with water to achieve the desired result.
Reminder: In chemistry, M is a measurement of concentration called Molarity, which denotes the moles of a substance per liter
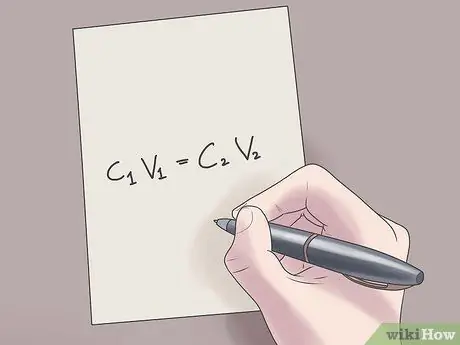
Step 2. Plug your values into the formula C1V1 = C2V2.
In this formula, C1 is the initial concentration of the solution, V1 is the volume of the initial solution, C2 is the final concentration of the solution, and V2 is the volume of the final solution. Plugging known values into this equation will help you find unknown values with less difficulty.
- You may find it helpful to put a question mark in front of the unit you want to search for to help you solve it.
-
Let's continue our example. We will enter the values we know as follows:
- C1V1 = C2V2
- (5 M)V1 = (1 mM)(1 L). Our two concentrations have different units. Let's stop here and move on to the next step.

Step 3. Consider any unit differences
Since solutions involve changes in concentration (which can sometimes be quite large), it's not uncommon for the two variables in your equation to have different units. While this is easy to overlook, unequal units in your equation can cause your answer to be incorrect. Before finishing, convert all values with different concentration and/or volume units.
-
In our example, we use different units for concentrations of M (molars) and mM (millimolars). Let's change our second measurement to M:
- 1 mM × 1 M/1,000 mM
- = 0.001 M

Step 4. Finish
Once all units are equal, solve your equation. This can almost always be done with simple algebra.
-
We stop our example problem here: (5 M)V1 = (1 mM)(1 L). Let's find the value of V1 with our new unit.
- (5 M)V1 = (0.001 M)(1 L)
- V1 = (0.001 M)(1 L)/(5 M).
-
V1 = 0.0002 L, or 0.2 mL.

Step 5. Understand how to use your answer correctly
Let's say you've found your missing value, but you're not sure how to use this new information in the actual dilution you need to do. This is understandable - the language of math and science sometimes doesn't match the real world. When you know the four values in the equation C1V1 = C2V2, do the dilution as follows:
- Measure the volume V1 from a solution with a concentration of C1. Then, add sufficient diluent (water, etc.) to make the total volume V2. This new solution will have the concentration you want (C2).
- In our example, for example, we first measure 0.2 mL of a 5 M solution. Next, we will add enough water to increase the volume of the solution to 1 L: 1 L - 0.0002 L = 0.9998 L, or 999, 8 mL. In other words, we will add 999.8 mL of water to our small sample solution. Our new, diluted solution has a concentration of 1 mM, which is our desired concentration.
Method 2 of 2: Making a Simple and Practical Dilution Solution

Step 1. Read any packaging for information
There are a variety of reasons why you might want to make a dilution solution at home, in the kitchen, or in another non-chemical laboratory. For example, making orange juice from a simple concentrate is a dilution. In many cases, the product that needs to be diluted contains information about the dilution that needs to be made, somewhere on the packaging. They may have precise instructions to follow. Here are some things to look for when looking for information:
- Product volume used
- Volume of diluent used
- Type of diluent used (usually water)
- Special mixing instructions
- You may not see information about the exact concentration of the liquid used. This information is not useful to the average consumer.

Step 2. Add the substance that acts as a diluent to the concentrated solution
For simple household dilutions, like the ones you might make in the kitchen, you really only need to know the volume of concentrate you're using and the approximate final concentration you want before you start. Dilute the concentrate with an appropriate amount of diluent, which is determined depending on the volume of the initial concentrate used. See below:
- For example, if we wanted to dilute 1 cup of orange juice concentrate to 1/4 its initial concentration, we would add 3 cups water into the concentrate. Our final mixture will have 1 cup of concentrate in 4 cups of all liquids - 1/4 of the initial concentration.
- Here's a more complicated example: If we wanted to dilute 2/3 cup of concentrate to 1/4 its initial concentration, we'd add 2 cups of water, because 2/3 cup equals 1/4 times 2&2/3 cup of the whole liquid.
- Be sure to add your substance in a container large enough to hold the final volume you want - a large bowl or similar container.

Step 3. Ignore the powder volume in most cases
Adding a powder (such as certain drink mixes) to a liquid is usually not considered a dilution. The volume change resulting from the addition of a small amount of powder to the liquid is usually small enough to be negligible. In other words, when adding a small amount of powder to a liquid, just add the powder to your desired final volume of liquid and mix.
Warning
- Follow any safety guidelines provided by the manufacturer or required by your company. This is especially important if you have to dilute an acid solution.
- Working with acidic solutions may require more detailed safety measures and guidelines than with non-acidic solutions.






