- Author Jason Gerald gerald@how-what-advice.com.
- Public 2024-01-19 22:11.
- Last modified 2025-01-23 12:04.
Chemists, biologists, environmentalists, and laboratory technicians all use pH to measure the acidity and alkalinity of a solution. The pH meter is a very useful and most accurate tool for measuring pH levels. To ensure you get the most accurate pH reading possible, there are many simple steps, from preparing the material to calibrating and testing it methodically. You can also measure the pH of the water using a special method.
Step
Part 1 of 3: Calibration Preparation

Step 1. Turn on the pH meter
Before you calibrate and use the pH meter, you need to turn it on and allow enough time for the meter to be ready for use. It usually takes about 30 minutes, but to make sure it's the right time, check the pH meter operating manual.

Step 2. Clean the electrodes
Remove the electrode from the storage solution and clean it with pure water in an empty beaker. After cleaning, dry with a tissue.
- Make sure you clean the electrodes in a beaker that is different from the beaker from which it was calibrated.
- Avoid rubbing the electrodes as these components have a sensitive membrane around them.
- If the electrodes are slightly dirty, check the operating manual for the recommended cleaning method.

Step 3. Prepare a buffer solution
In general, you will need more than one buffer solution to calibrate the pH meter. The first is a “neutral” buffer solution with a pH of 7, and the second one whose pH is close to the pH of the sample, either pH 4 or 9.21. A buffer solution with a higher pH (9.21) should be calibrated to measure alkalinity, whereas a buffer with a higher pH (9.21) should be calibrated to measure alkalinity. Low pH (4) should be used to measure acid samples. After selecting the buffer solution, allow it to reach the same temperature, as pH readings are temperature dependent. Pour the buffer solution into a separate beaker for the calibration process.
- Seek information from the manufacturer of the pH meter or a professional or educational institution on how to obtain a pH buffer solution.
- The buffer solution must be put in a beaker for a maximum of two hours.
- Do not pour used buffer solution into the original container.
Part 2 of 3: Calibrating the pH Meter

Step 1. Insert the electrode into a buffer solution with a pH of 7 and start taking the reading
Press the measure or calibration button to start reading the pH after the electrode is placed in the buffer solution.
Allow the pH reading to stabilize by allowing it to stand for about 1-2 minutes

Step 2. Adjust the pH
After obtaining a stable reading, set the pH meter to the pH value of the buffer solution by pressing the measure button a second time. Adjusting the pH meter after a stable reading will result in a more accurate and appropriate reading.
Although it's not necessary, if you stir the buffer solution before measuring, make sure you mix the entire buffer and sample in the same way

Step 3. Clean the electrodes with pure water
Clean and dry with a lint-free cloth before using the other buffer solution.

Step 4. Insert the electrode into the buffer solution with a pH value of 4 and start the reading
Press the meter button to start the pH reading after the electrode is placed in the buffer solution.
If you are not using a buffer solution with a pH value of 4 for calibration, use a buffer solution with a pH value of 9.21

Step 5. Adjust the pH a second time
Once the reading is stable, set the pH meter to the pH value of the buffer solution by pressing the measure button.
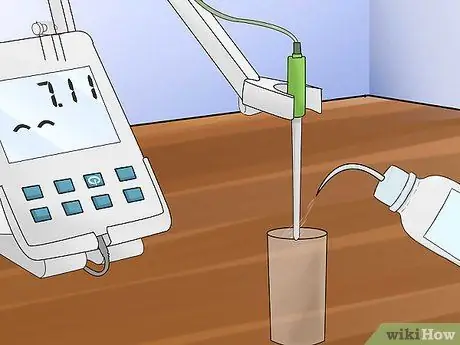
Step 6. Clean the electrodes
Use pure water for cleaning. Use a lint-free tissue to dry the electrode before using it on another buffer solution.
Part 3 of 3: Using a pH Meter

Step 1. Insert the electrode into the sample and start reading
After the electrode is inserted into the sample, press the measuring button and leave the electrode in the sample for about 1-2 minutes.

Step 2. Determine the pH level
Once the reading is stable, press the measure button. This is the pH level of your sample.

Step 3. Clean the electrodes after use
Rinse the electrodes with purified water and dry with a lint-free tissue. You can store the pH meter after it is clean and dry.
Check the operating manual to find out the proper storage method for your pH meter
Tips
- Make sure you always ask if you are unsure about a process. Check with the laboratory supervisor or check in the operating manual at home.
- All pH meters are not much different. Check all necessary guidelines before starting to calibrate and use the pH meter.






