- Author Jason Gerald gerald@how-what-advice.com.
- Public 2023-12-16 10:50.
- Last modified 2025-01-23 12:04.
Spectrophotometry is an experimental technique used to measure the concentration of a solute in a particular solution by calculating the amount of light absorbed by that substance. This technique is very useful because certain compounds will also absorb different wavelengths of light at different intensities. By analyzing the light passing through a solution, you can identify the compounds dissolved in the solution and their concentrations. The tool used to analyze solutions with this technique in the laboratory is a spectrophotometer.
Step
Part 1 of 3: Preparing the Sample

Step 1. Turn on the spectrophotometer
Most spectrophotometers need to be warmed up before they can give accurate measurements. So, start the machine and then let it sit for at least 15 minutes before measuring the sample.
Use this time to prepare the sample
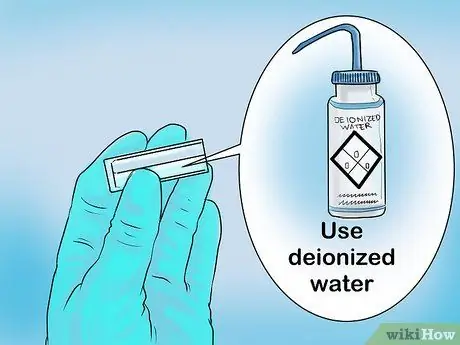
Step 2. Clean the cuvette or test tube
In school laboratories, disposable test tubes may be available which do not have to be cleaned first. However, if you are using an ordinary cuvette or test tube, be sure to clean the apparatus thoroughly before use. Rinse all cuvettes with deionized water.
- Be careful using cuvettes as they are quite expensive.
- While using the cuvette, do not touch the side where the light passes (usually the clear side of the container).
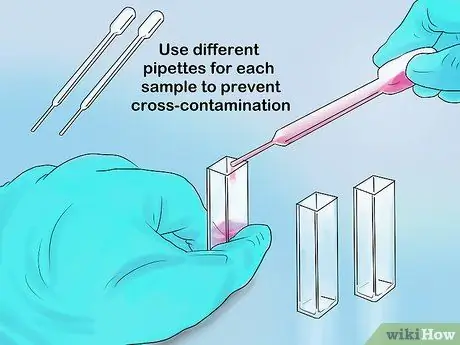
Step 3. Pour sufficient sample into the cuvette
The maximum volume of part of the cuvette is 1 ml, while the maximum volume of the test tube is 5 ml. Your measurements should be accurate as long as the spectrophotometer's light can still pass through the sample and not an empty part of the container.
If you are using a pipette to insert samples, use a new tip for each sample. That way, cross-contamination can be avoided

Step 4. Prepare the control solution
These solutions which are also known as blanks or blanks contain only the solvent in the solution being analyzed. For example, if you have a sample of salt dissolved in water, the blank solution you need is water. If the water you are using is red, you should also use a red blank solution. Use a similar container to hold the blank solution in the same volume as the sample.
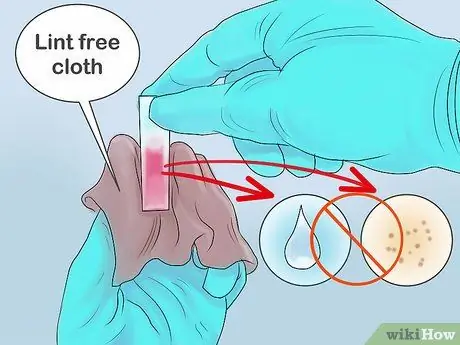
Step 5. Wipe the outside of the cuvette
Before inserting the cuvette into the spectrophotometer, you must ensure that it is clean to avoid interference with the measurements due to dust particles or impurities. Use a lint-free cloth to remove any water droplets or dust adhering to the outside of the cuvette.
Part 2 of 3: Experimenting
Step 1. Determine and adjust the wavelength of light to analyze the sample
Use a single wavelength of light (monochromatic beam) to increase measurement effectiveness. Choose the color of light that can be absorbed by the chemical content that is thought to be dissolved in the test sample. Set the wavelength according to the specifications of the spectrophotometer you are using.
- In school laboratories, these wavelengths will usually be given in the experimental instructions.
- Because the sample will reflect all visible light, the wavelength of the experimental light's color is usually always different from that of the sample.
- An object appears a certain color because it reflects a certain wavelength and absorbs all other colors. Grass appears green because the chlorophyll in it reflects green and absorbs other colors.
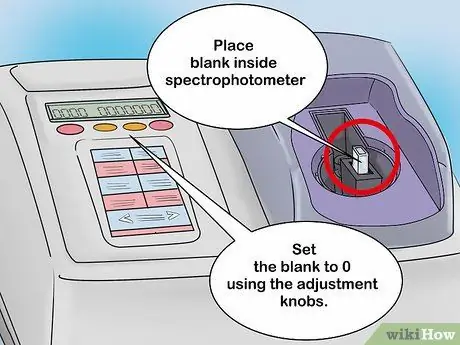
Step 2. Calibrate the spectrophotometer with a blank solution
Place the blank solution into the cuvette holder and close the spectrophotometer. On the analog spectrophotometer screen, there is a needle that will move based on the intensity of light detection. After the blank solution is inserted, the needle should move to the right. Record this value in case you need it later. Allow the blank solution to remain in the spectrophotometer, then slide the needle to zero using the adjusting knob.
- Digital spectrophotometers can also be calibrated in the same way. However, this tool is equipped with a digital screen. Set the reading of the blank solution to 0 with the control knob.
- Even if the blank solution is removed from the spectrophotometer, the calibration will still be valid. So, when you measure the whole sample, the absorbance of the blank will be automatically reduced.

Step 3. Remove the blank and test the spectrophotometer calibration results
Even after the blank solution is removed from the spectrophotometer, the needle or number on the screen should still read 0. Place the blank solution back into the spectrophotometer and make sure the reading does not change. If the spectrophotometer is calibrated properly using a blank solution, the result on the screen should still be 0.
- If the needle or the number on the screen does not read 0, repeat the calibration step with a blank solution.
- If the problem persists, seek help or have someone check the spectrophotometer.

Step 4. Measure the absorbance of the sample
Remove the blank solution and insert the sample into the spectrophotometer. Wait about 10 seconds for the hands to stabilize or the numbers on the digital display stop changing. Record the percentage transmittance and/or absorbance of the sample.
- The more light that is passed, the less light is absorbed. Usually, you need to record the absorbance value of the sample which is generally expressed as a decimal number, for example 0.43.
- Repeat the measurement of each sample at least three times and then calculate the average. That way, the results you get will be more accurate.

Step 5. Repeat the experiment with different wavelengths of light
Your sample may contain several compounds that have different absorbances depending on the wavelength of the light. To reduce uncertainty, repeat sample measurements at 25 nm wavelength intervals across the light spectrum. This way, you can detect other dissolved chemicals in the sample.
Part 3 of 3: Analyzing Absorbance Data

Step 1. Calculate the transmittance and absorbance of the sample
The transmittance is how much light can pass through the sample and reach the spectrophotometer. Meanwhile, absorbance is how much light is absorbed by one of the dissolved chemicals in the sample. There are many modern spectrophotometers giving output in the form of transmittance and absorbance. However, if you get a light intensity value, you can also calculate these two values yourself.
- The transmittance (T) can be determined by dividing the intensity of light passing through the sample solution by the amount of light passing through the blank solution. This value is usually expressed as a decimal number or a percentage. T = I/I0, where I is the sample intensity and I0 is the blank intensity.
- Absorbance (A) is expressed as a negative base 10 logarithm (exponent) transmittance: A = -log10T. So, if T = 0, 1, A = 1 (0, 1 is 10 to the power of -1). This means that 10% of the light is passed, while 90% is absorbed. Meanwhile, if T= 0.01, A = 2 (0.01 is 10 to the power of -2). This means, the light that is passed is 0.1%.
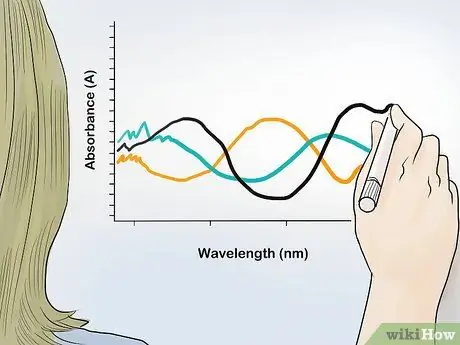
Step 2. Graph the absorbance value vs. wavelength
Express the absorbance value as the y-axis and the wavelength as the x-axis. From the dots of all absorbance results in each wavelength, you will get the absorbance spectrum of the sample, and identify the content of the compound and its ratio in the sample.
Absorbance spectra usually have peaks at certain wavelengths. These peak wavelengths allow you to identify specific compounds
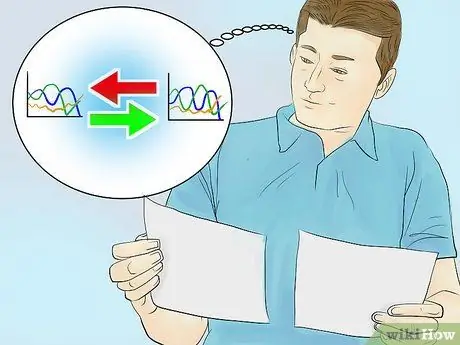
Step 3. Compare your absorbance spectrum with a graph of a known compound
Each compound has a unique absorbance spectrum and always has the same peak wavelength in each measurement. By comparing the graph you get with the graph of a particular known compound, you can identify the solute content in the sample solution.






