- Author Jason Gerald gerald@how-what-advice.com.
- Public 2023-12-16 10:50.
- Last modified 2025-01-23 12:04.
If you find the periodic table confusing and difficult to understand, don't worry, you are not alone! Understanding how the periodic table works can be difficult, but by learning how to read it, you will be successful in your science studies. Start by understanding the structure on the periodic table and the information it shows about the elements. Next, you can study each of the elements. Finally, use the information listed on the periodic table to find out the number of neutrons in an atom.
Step
Part 1 of 3: Understanding the Structure of the Periodic Table
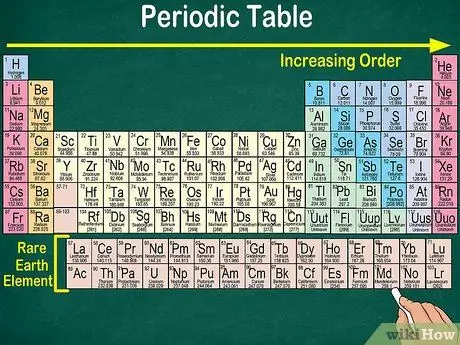
Step 1. Read the periodic table from top left to bottom right
The elements are arranged according to their atomic number. The further to the right and down, the higher the atomic number. The atomic number is the number of protons that an element's atom has. As you go further to the right, you'll also notice that the mass number of each atom increases. That is, you can understand the weight of an element even just by looking at its location on the table.
- The further to the right or down, the atomic mass of an element will increase because the atomic mass is calculated by adding up the protons and neutrons in each atom of the element. The number of protons increases with the element, which means its weight also increases.
- Electrons are not included in atomic mass because compared to protons and neutrons, electrons do not have much effect on atomic weight.

Step 2. Understand that each element contains 1 more proton than the atom to its left
You can tell this by looking at the atomic number. The atomic numbers are arranged from left to right. The elements are also separated into 3 groups, you can see the grouping in the table.
For example, the first row lists hydrogen, which has the atomic number 1, and helium, which has the atomic number 2. However, these two elements are located at the far left and right of the table because they are in different groups
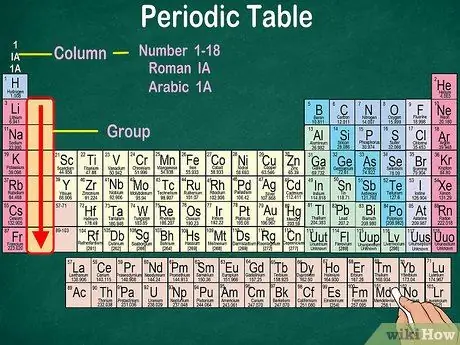
Step 3. Identify groups of atoms, which have the same physical and chemical properties
Groups are indicated by vertical columns. In most cases, groups are characterized by the same color. This helps you identify which elements have similar physical and chemical properties. This will make it easier for you to predict the reactions of these elements. Each element in a given group has the same number of electrons in its outermost orbital.
- Most elements only belong to one group. However, hydrogen can be classified as either a halogen or an alkali metal. In some tables, hydrogen appears in both groups.
- In most cases, the columns will be numbered 1-18, either at the top or bottom of the table. The numbers can be displayed in roman numerals (IA), arabic numerals (1A), or numbers (1).
- Read the atomic groups from top to bottom.
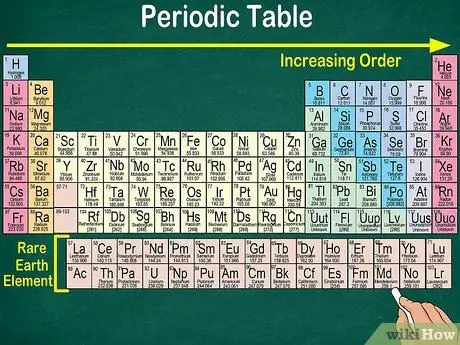
Step 4. Notice the blank space in the table
In addition to the atomic number, the arrangement of the elements into groups and groups also takes into account the same physical and chemical properties. This way, you will better understand how each element reacts. The addition of chemical elements makes their classification more difficult, so it is not surprising that the periodic table contains empty space.
- For example, the first 3 rows have blank spaces, because the transition metals that appear in the table are elements with atomic number 21.
- Similarly, elements 57 to 71, which are rare earth elements or rare earth elements, are depicted separately at the bottom right of the table.
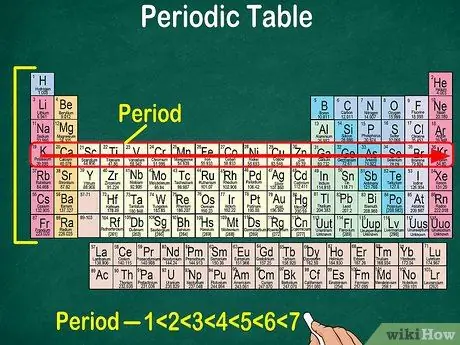
Step 5. Notice that each row is called a period
All elements in a period have the same number of atomic orbitals, through which electrons will pass. The number of orbitals will correspond to the number of periods. The periodic table shows 7 rows, which means there are 7 periods.
- For example, an element in period 1 has 1 orbital, while an element in period 7 has 7 orbitals.
- In most cases, the periods are numbered 1-7 from top to bottom on the left side of the table.
- Read the period of the elements following the row from left to right.
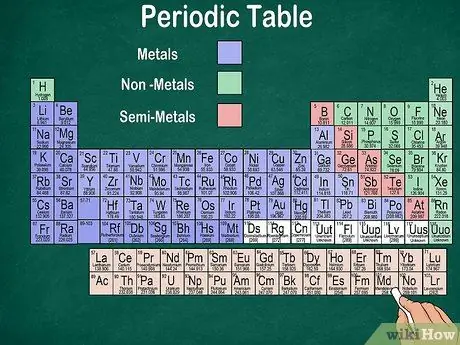
Step 6. Distinguish between metals, semimetals and nonmetals
You can better understand the properties of an element by recognizing the type of element. Fortunately, most of the periodic table uses color to indicate whether an element is a metal, a semimetal, or a nonmetal. You'll find metal elements on the right of the table, while non-metals on the left. The semi-metal group is located between metals and non-metals.
- Remember that hydrogen can be grouped with the halogens or the alkali metals because of its properties. Therefore, it is natural for hydrogen to appear on both sides of the table or be a different color.
- An element is called a metal if it is shiny, solid at room temperature, conducts heat and electricity, and is soft and elastic.
- An element is considered a nonmetal if it is not shiny, does not conduct heat or electricity, and is hard. These elements are usually gaseous at room temperature, but can also be solid or liquid at certain temperatures.
- An element is called a semimetal if it has the combined properties of a metal and a non-metal.
Part 2 of 3: Studying the Elements
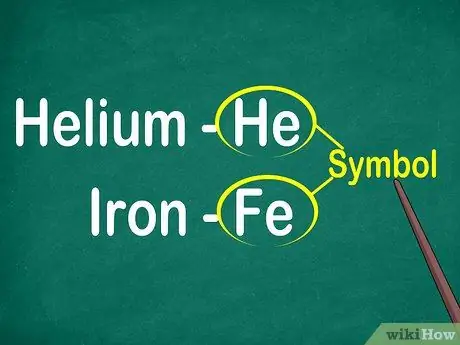
Step 1. Identify the 1 to 2 letter element symbols
The symbol is usually located in the center of a square with a large font. Symbols are abbreviations for element names, which have been standardized in various languages. When doing experiments or working on elemental equations, you will probably use element symbols. Therefore, like it or not, you have to familiarize yourself with the elemental symbols.
Symbols are usually derived from the Latin name of an element, but are sometimes derived from names that are widely used, especially new elements. For example, the symbol for Helium is He, which stands for this well-known name. However, the symbol for iron is Fe, which is relatively difficult to spot at first sight

Step 2. Find the full name of the element, if any
This is the name of the element that you will use if you have to write it down in full. For example, "Helium" and "Carbon" are the names of elements. In most cases, the element name is below the symbol, but the placement can vary.
Some periodic tables may not include the full name and only use symbols
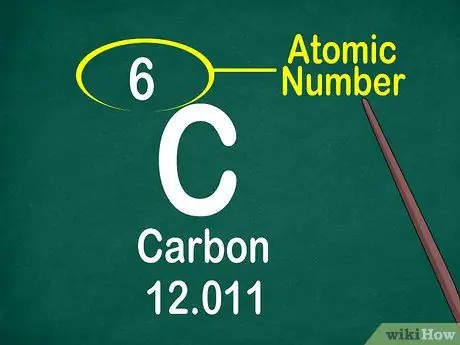
Step 3. Note the atomic number
The atomic number is usually located at the top of the box, either in the middle or in the corner of the box. However, the atomic number can also be located under the element symbol or element name. The atomic numbers are ordered from 1-118.
The atomic number is an integer, not a decimal
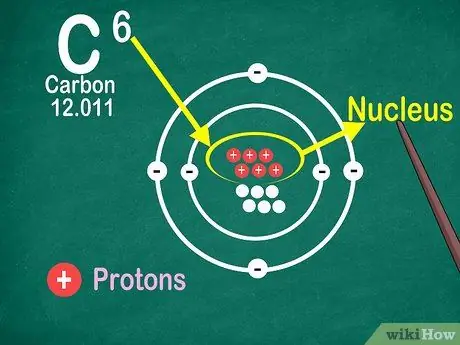
Step 4. Know that the atomic number is the number of protons in the atom
All atoms in an element have the same number of protons. Unlike electrons, protons cannot be captured or released by atoms. Elements will change if atoms can catch or lose atoms.
You also need the atomic number to find out the number of electrons and neutrons
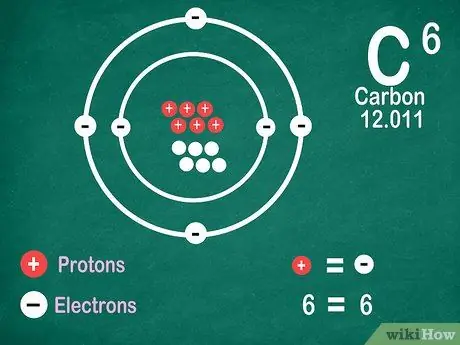
Step 5. Know that elements contain the same number of electrons as protons, unless the element undergoes ionization
Protons have a positive charge, while electrons have a negative charge. Since a neutral atom has no electric charge, it means that it has the same number of electrons and protons. However, atoms can lose and gain electrons, which makes them ionized.
- Ions are electric charges. If there are more protons in an ion, the charge is positive, which is a positive (+) sign next to the ion symbol. If the number of electrons in the ion is more, the charge is negative, which is negative (-).
- You will not see a positive or negative sign if an atom is not an ion.
Part 3 of 3: Using Atomic Weight to Count Neutrons

Step 1. Know the atomic weight
The atomic weight is usually located at the bottom of the box, below the element symbol. Atomic weight is the combined weight of the particles in the atomic nucleus, including protons and neutrons. However, ions can complicate the counting process. Thus, the atomic weight indicates the average atomic mass of the element and the atomic mass of its ions.
- Because of their average weight, most atoms have a decimal atomic weight.
- Although the weight of an element looks as if it increases from left to right, that's not always the case.
Step 2. Determine the mass number of the element you are studying
You can find the mass number by rounding the atomic mass. This fact proves that atomic weight is the average of all atomic masses, including ions.
For example, the atomic weight of carbon is 12,011 so it is rounded up to 12. Likewise, the atomic weight of iron is 55.847 so it is rounded up to 56
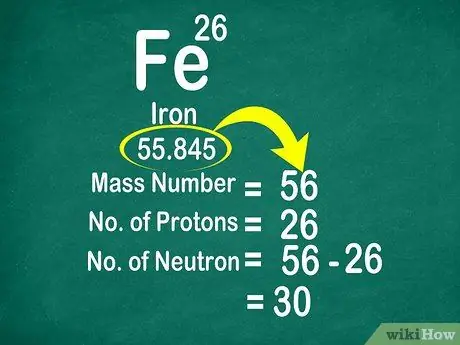
Step 3. Subtract the mass number from the atomic number to count the neutrons
The mass number can be calculated by adding the number of protons to the number of neutrons. This will make it easier for you to calculate the number of neutrons in an atom, by subtracting the mass number from the number of protons
- Use this formula: Neutron = Mass Number - Proton
- For example, the mass number of Carbon is 12 and has 6 protons. Thus, we can know that Carbon has 6 neutrons because 12 - 6 = 6.
- Another example, the mass number of iron is 56 and has 26 protons. Thus, we know that iron has 30 neutrons because 56 - 26 = 30.
- Isotopes of atoms contain different numbers of neutrons so their atomic weights change.
Tips
- Reading the periodic table is difficult for some people. Don't be discouraged if you find it difficult to study the periodic table!
- The colors in the table may vary, but the content remains the same.
- Some periodic tables may provide incomplete information. For example, some tables only give the symbol and atomic number. For that, look for a table that suits your needs!






