- Author Jason Gerald gerald@how-what-advice.com.
- Public 2023-12-16 10:50.
- Last modified 2025-06-01 06:05.
Videos showing that Mountain Dew (a carbonated soft drink produced by the Pepsi company) glow by mixing hydrogen peroxide and baking soda is a hoax. To actually make a glow stick (a plastic tube filled with a liquid chemical that ignites when it reacts) without breaking the finished glow stick and transferring its contents into the tube (this method is called deception), you have to show your scientific side (also prepare a fee). required). If you are still curious, continue reading this article. This activity is fun for anyone.
Step
Method 1 of 2: Using TCPO (Dual Color)

Step 1. Wear clothing to protect your eyes and skin
These chemicals are carcinogenic (causing cancer) and are very dangerous if used inappropriately. You should also be aware that the chemicals needed are very expensive, and usually tend to discourage you from buying them unless you're going to be making a lot of glow sticks. At a minimum you should have:
- Latex gloves
- Ventilated eye protection (laboratory goggles)
- Long sleeve shirt/shirt
- Mask
- Clean and tidy work environment
- Clean glass container/tube with lid
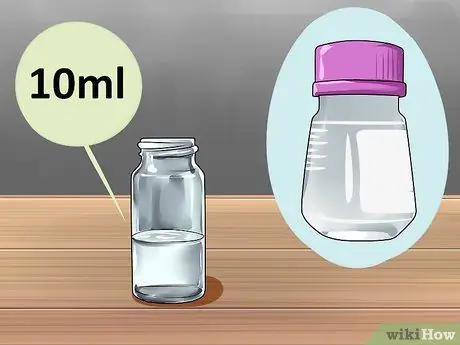
Step 2. Start with 10 mL of diethyl phthalate (DP) solution
The DP solution is the basic ingredient and makes up the bulk of the liquid in the glow stick that you are going to make. The solution will hold the chemicals that actually ignite and solidify them into lighter colors. Start with 10 mL DP, but be aware that you can double it for a larger glow stick size, or halve it for a smaller glow stick. This solution looks like water, but a chemical reaction is required to produce a glow that doesn't work in water.
- To get the chemical, you may have to try to find it through online sales (note: on the websites of reputable research/chemical suppliers of chemicals). In America, for example Alfa Aesar and Sigma Aldrich.
- Do not attempt to replace DP with water. Although DP doesn't technically "do" anything in the reaction, it is needed to allow light to be created. Meanwhile, water will actually block the release of the light beam.
- If you want to double the amount of solvent, you should also double the other ingredients.

Step 3. Add 3 mg of the fluorescent dye of your choice to add color
You can not using ordinary dyes or dye additives; make sure you use a fluorescent dye. The various dyes will not be the same immiscible colors as they are, when they glow. So to produce colors, trust the instructions given below. There are different types of dyes that you can use, depending on the color you want:
- 9, 10- bis(phenylethynyl)anthracene for color green
- Rubren e for color yellow
- 9, 10- diphenylanthracene for color blue
- Rhodamine B for color Red (note: rhodamine dissipates quickly, meaning the red color tends to fade quickly)
- To produce color White Mix each half of the yellow and blue colors.
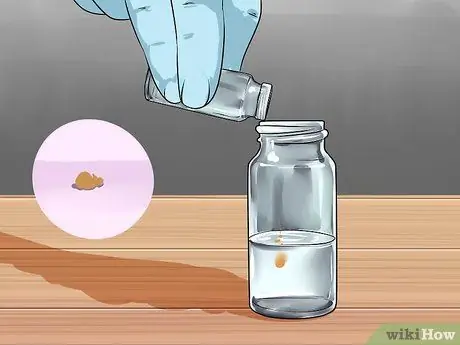
Step 4. Add 50 mg TCPO to the mixture
TCPO is the abbreviation/symbol for bis(2, 4, 6 -trichlorophenyl)oxalate, which you may want to know before buying. They are relatively expensive, but you can make them quite cheap if, again, you are experienced and competent with chemicals. On the other hand, making it yourself is not recommended.
- In this way, TCPO is used to replace luminol-the component that makes the mixture glow and lasts for several hours.
- TCPO is truly carcinogenic, and should be handled with care. never ever inhale TCPO.

Step 5. Add 100 mg sodium acetate to the mixture
If you don't have sodium acetate on hand, a mixture of sodium bicarbonate (baking soda) and sodium salicylate can be used as an effective substitute. After adding the solution, close the bottle and shake it.

Step 6. As a final step, add 3 mL of 30% hydrogen peroxide to the mixture
It is very important to do this step at the end, because this material causes a chemical reaction. Recap the bottle, shake it properly, and finally the light will come on. You should have a very impressive glow stick case/tube/glow stick.
- Hydrogen peroxide functions as a catalyst (activating agent), not part of the reaction that occurs. So you don't need too much of it.
- If you buy a glow stick, you will need to break it to make the tube glow. "Crack" is the sound you hear when you break a small glass tube/bottle containing hydrogen peroxide.
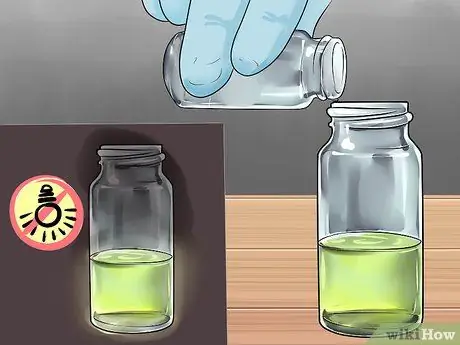
Step 7. Add more TCPO and Sodium Acetate to make the reaction last longer
If you're passionate about it, mess up the test site with the recipe to see what it takes to guarantee the best results. The reaction occurs because when combined, TCPO and Sodium Acetate will release energy, until both begin to decrease. The energy is picked up by the fluorescent dye, which converts the energy into light. The more quantities of each, the more energy is formed and the reaction takes longer.
Method 2 of 2: Using Luminol

Step 1. Wear protective eyewear
In addition, wear gloves to protect your skin. It's also a good idea not to wear your best clothes or what you usually wear to worship. Choose old clothes or wear layering over the clothes you want to protect. Some of the ingredients are dangerous-this experiment is not intended for children!
For children, note: you will be working with a solution that has a pH/acidity level close to 12 (pH values range from 0 to 14). It basically means don't swallow it, don't put it in your eyes, don't bathe in it, and don't expose your body to it directly. Understand? Continue
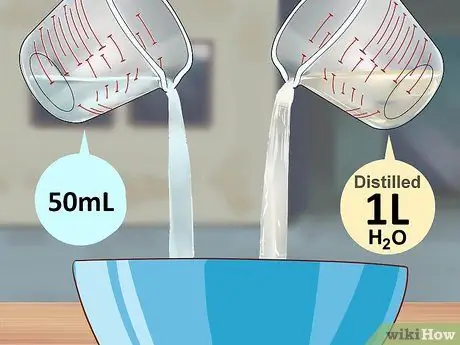
Step 2. Combine 50 milliliters of hydrogen peroxide and one liter of distilled water in a bowl for mixing
Ceramic bowls are best, but plastic bowls will work too. Use a funnel, measuring tube, and baster (a glass tube like a pipette with a ball-shaped head made of rubber, used to suck/inject liquids) to keep all ingredients properly measured and away from you.
Hydrogen peroxide is used to replace the nitrogen atoms of the luminol with oxygen. When that happens, all the chemicals create a (out of control) noise and start to “party” and electrons fly all over the place and what is the result? Yes, light
Step 3. Mix 0.2 grams of luminol, 4 grams of sodium carbonate, 0.4 grams of copper sulfate, 0.5 grams of ammonium carbonate and one liter of distilled water in a second bowl
Very important for no touch the luminol. Use a funnel so that everything goes safely and easily. Unfortunately, these hazardous chemicals will not float freely in the air like the graphic presented.
- Unless you're a coroner or one of those crazy spies/criminologists, chances are you don't have this piece of equipment lying around the house (hopefully not…). If you're having trouble starting a glow stick business (worst idea ever), try to find a reputable research/chemical chemical supplier website for materials supply. In America, for example Alfa Aesar or Sigma Aldrich.
- Mix all ingredients well. Don't use your hands - use some metal or plastic household utensils.

Step 4. Clean the container and dry it completely
It is very important to use a clean tube to make glow sticks. The last thing you need is for the other ingredients to interact with a reaction that will depend on how you make the chemicals glow.

Step 5. Next, attach the lids of each container properly
This allows you to quickly close them after the charging stage. Not that the light will appear and leave you, but that it will stay where it is.

Step 6. Mix equal amounts of the first and second solution into the container, then close the lid
Shake as soon as the lid is closed tightly. Next, turn off the lights!
If in this way the light does not radiate, an error has occurred. Repeat one more time

Step 7. Watch as the chemical mixture produces colorful lights
Bring your glow stick to the party and charge your friends to play it. But act quickly because the light won't last long. Are your hopes shattered? Do the second way to help!
The reaction that luminol and hydrogen peroxide creates doesn't last long-perhaps only about two minutes. To make it last up to several hours, consider the following method (which is much easier and possible if you do it in a laboratory, but is still quite relevant)
Tips
- Luminol is the chemical that makes blood glow. These chemicals can be found in stores selling research/laboratory materials and supplies, on the Internet or in children's espionage kits.
- Sodium carbonate, ammonium carbonate, and copper sulfate pentahydrate are white powders. All of these materials can be found in stores that sell research/laboratory materials and supplies.
- This process can lead to a mess. Spread a sheet of newspaper or do this activity in an area that you can easily clean afterwards.
- It's much easier and cheaper to buy finished glowsticks, unless you're going to be buying these products in bulk.
Warning
- Always wear protective eyewear when you work with chemicals.
- Children must be supervised when using glow sticks. It is possible that children will try to break the glow stick and play with or digest/eat the contents, which can cause serious injury.
- These instructions are how to make a very serious/heavy glow stick. Nothing is more simply described because it does not exist. Leave the making of the glow sticks to the experts if you're only going to see chaos all around you-they're dangerous ingredients.
- Put on gloves. Do not touch the luminol or eat it.
-
Copper sulfate is a toxic material. Once again: be careful.






